O.O & T.K are co-first authors.
Clonal (myelo) hematopoiesis of indeterminate potential (CHIP) arises from somatic mutations in myeloid genes, providing a proliferative and survival advantage to the affected “CHIP-initiating” early hematopoietic precursor cells. Understanding the conditions that lead to the emergence of detectable clones (early seeding of myeloid neoplasia-MN) is of great interest due to its clinical implications. Among CH facilitating states, aging has been extensively studied, but a clear distinction between the impact of chronological versus biological aging remains challenging. Additionally, other factors contributing to CHIP development may include chemotherapy, genetic predisposition, bone marrow failure states, or immunodeficiency. However, not all CHIP-promoting factors have been identified because they are subtle, pathophysiologically complex, and thus remain cryptic.
We hypothesized that episodes of emergency hematopoiesis (EH), such as those caused by acute infections, could potentially result in acquisition of CHIP with repeated episodes of EH resulting in clonal expansion. If this hypothesis is confirmed, one could envision that both instances of significant hematopoietic expansion (high leukocytosis) and repeated cycles of EH could be at play in conjunction with chronological factors. For instance, the older an individual is, the greater is the number of EH episodes possible.
To examine our theory, we prospectively collected blood samples from patients admitted at the intensive care unit (ICU) of Cleveland Clinic and Yale University. Inclusion criteria required a white blood cell (WBC) count ≥ 15K/uL. Patients with known or prior malignancies and those exposed to chemotherapy were excluded. For relevant comparisons, we assessed CH incidence in our cohort against a group of patients with solid tumors screened for CH (n=107) but without acute infections, along with historical CHIP cohorts. CH screening employed a targeted NGS myeloid gene panel with a reported VAF cut-off of 2%.
A total of 78 individuals admitted to the ICU with possible infections and leukocytosis were enrolled in this study. The median age was 70 years (IQR: 61-77), with a M:F ratio of 1.7 (Table 1). Time from leukocytosis onset to sampling was 3 days (IQR: 1-5), the median WBC at ICU admission was 18 K/uL (IQR: 16-21), and the median absolute neutrophil count (ANC) was 16 K/uL (IQR: 12-20).
Overall, 94 variants were identified including 16 (median VAF 50.1) variants of potentially germline origin, and 4 variants of unknown significance. We observed 74 CHIP-associated mutations in 37 (47%) patients at a median VAF of 14% (4-47%). The landscape of CHIP mutations included alterations in DNMT3A (20%), TET2 (12%), ASXL1 (12%), SMC1A, TP53, and PPM1D genes (6% each). Although higher rates of CHIP were expected in the cancer cohort, no difference was found between the two groups (40% vs. 47% in the ICU, p=0.3). DNMT3A, ASXL1 and PPM1D mutations were more frequently encountered in the cancer cohort (Table 1).
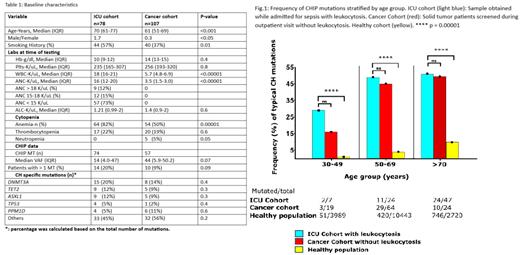
The prevalence of CHIP in the ICU cohort was higher in older age groups: 30-39 (29%), 50-69 (49%), and ≥70 (51%), compared to the expected frequency in comparable age ranges (and median range) in a historical healthy population, which was 1%, 4%, and 10%, respectively (<0.00001; Fig.1). Notably, CHIP was more prevalent among smokers (57%; p=0.003). No significant differences were found when comparing CHIP + and CHIP - patients of the ICU cohort with regards to WBC (median 18K/uL both). Similarly, CHIP prevalence did not change according to the degree of leukocytosis in an analysis where patients were ranked by WBC (≥18K/uL at 44% vs. <18K/uL at 46%). When looking at the impact of CHIP on critical care outcomes, a logistic regression model accounting for age, gender, and mutation type revealed that the presence of DNMT3A mutations was associated with a higher risk of diagnosis of overt septic shock (log OR= 2.4, 95% CI 0.19-5.4, p<0.05).
In summary, our prospective observational study has shown that sepsis is associated with preferential mutant clone expansion, primarily due to EH rather than the extent of leukocytosis and may be contributing to the acquisition of new clones. Whether such episodes result in permanent acquisition of new CHIP mutations with potential for MN progression will be the subject of longitudinal analysis of these samples, and findings will be presented at the ASH meeting.
Disclosures
Singh:Rigel: Other: Advisor or review panel participant. Maciejewski:Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal